Matter
If you look around you, you will find millions of matter. A book is a matter, a pen is a matter, a chair is a matter. Matter is anything that occupies space. Matter is composed of similar or different elements, either singular or combined with each other. Scientists have identified over 110 different elements. Each element differs from others in the composition of its atoms. Every element consists of tiny particles called atoms, and an atom of one element differs from an atom of another element in the number of protons, neutrons, and electrons. An element exists in nature as individual atoms or combined with itself or others. When it is combined with itself or others, it is then called molecules.
Atoms
How does a copper wire differ from a piece of iron?
Copper and iron are different matter, each called an element. Scientists have identified over 110 different elements, such as aluminum, carbon, oxygen, hydrogen, and others.
An element consists of tiny particles that distinguish it from other elements, and these particles are called atoms.
Atomic Concept
An atom is the smallest unit of an element that possesses its distinctive properties, distinguishing it from other elements. Atoms are extremely small particles that cannot be seen with a compound optical microscope. However, there are special microscopes that enable us to observe their arrangement.
Components of an Atom
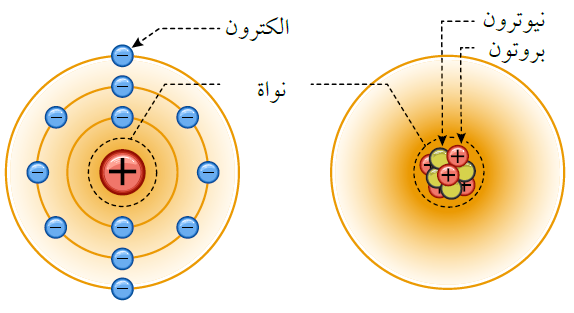
An atom of an element consists of three types of particles:
- Protons: Positively charged particles.
- Neutrons: Neutrally charged particles. Protons and neutrons are located in the nucleus of the atom.
- Electrons: Negatively charged particles.
Electrons orbit around the nucleus of the atom.
Identity of an Element
Atoms of different elements differ in the number of protons. There are no two elements with the same number of protons in their atoms.
For example:
The carbon nucleus contains 6 protons, while the oxygen nucleus contains 8 protons.
Arrangement of Atoms
Atoms of different elements are arranged in specific patterns, which affect their properties, characteristics, and uses.
Example: Diamond and graphite:
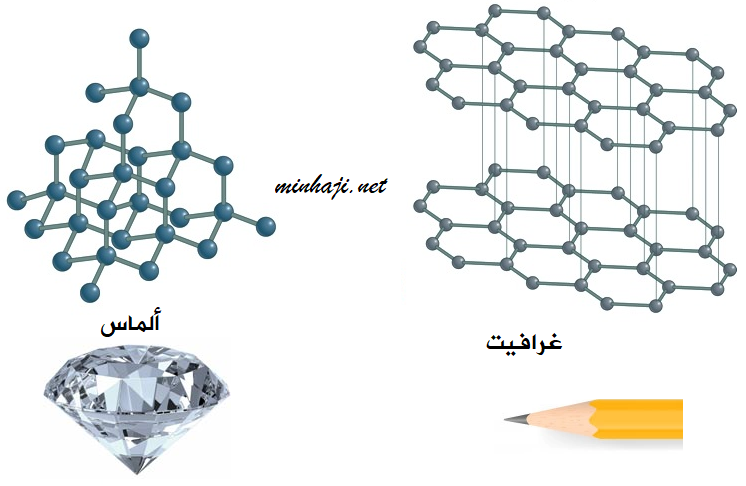
Both diamond and graphite are composed of the element carbon, but they differ in the arrangement of atoms.
Graphite:
- The carbon atoms in graphite are arranged in parallel layers.
- Characteristics of graphite: Black color, soft, easily breakable.
- Use: Production of pencils.
Diamond:
- The atoms in diamond are arranged in a tetrahedral structure.
- Characteristics of diamond: High hardness.
- Use: Jewelry production.
Molecules
Concept of an Element
An element is a pure substance composed of one type of atom that cannot be broken down into simpler substances by chemical or physical means.
Some elements exist in the form of:
Individual atoms, such as gold (Au) and aluminum (Al).
Molecules, such as oxygen (O2), hydrogen (H2), and water (H2O).
Components of Molecules
A molecule is formed by the union of two or more atoms of the same or different types through electron sharing.
Examples: A hydrogen molecule consists of the union of two hydrogen atoms and is written as (H2).

An oxygen molecule consists of the union of two oxygen atoms and is written as (O2).

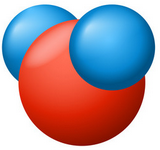
A water molecule consists of the union of two hydrogen atoms with one oxygen atom and is written as (H2O).
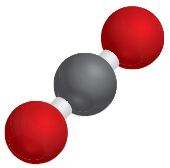
A carbon dioxide molecule consists of the union of two oxygen atoms with one carbon atom and is written as (CO2).
How do molecules differ from each other?
Molecules differ from each other in the number and type of constituent atoms.
إعداد : شبكة منهاجي التعليمية
03 / 07 / 2023
النقاشات